...............................................................................
The Benzene Molecule
What is the geometry of benzene?
The chemical compound benzene (C6H6) is a colorless, flammable,
aromatic hydrocarbon, that is a known carcinogen.
It boils at 80.1°C and melts at
5.5°C.
Benzene has a heat of
vaporization of 44.3 kJ/mol and a heat of fusion of 9.84 kJ/mol.
Produced by hydrogen reduction of
some allotropes of carbon, or from petroleum, it is used in the creation of
drugs, plastics, gasoline, synthetic rubber, napalm and dyes.
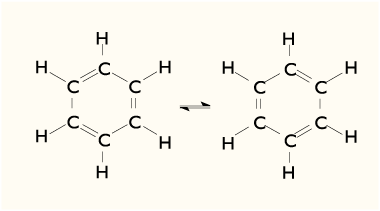
The benzene molecule is composed of six carbon atoms joined in a
ring with one hydrogen atom attached to each. As it contains only carbon and
hydrogen atoms, benzene is classed as a hydrocarbon.
X-ray diffraction shows that all six carbon-carbon
bonds in benzene are of the same length, at 1.4A.
You can check this measurement below using Jsmol. The
C–C bond lengths are greater than a double bond (1.35 A) but shorter than a
single bond (1.47 A).
This intermediate distance is consistent with electron
delocalization: the electrons for C–C bonding are distributed equally between
each of the six carbon atoms.
Benzene has 6 hydrogen atoms – fewer than the
corresponding parent alkane, hexane.
The molecule is in a flat or planar hexagon ring. The
molecular orbital description involves the formation of three delocalized π
orbitals spanning all six carbon atoms, while the valence bond description
involves a superposition of resonance structures.
It is likely that this stability contributes to the
peculiar molecular and chemical properties known as aromaticity.
To accurately reflect the nature of the bonding,
benzene is often depicted with a circle inside a hexagonal arrangement of
carbon atoms.
Cyclohexane, is also a six carbon
ring structure but instead contains all single bonds and is not a flat ring.
The ring formation of cyclohexane
attempts to attain the bond angles for the tetrahedral carbon atoms.


No comments:
Post a Comment