.................
Turbidity
Water is clarified using the
processes of coagulation and flocculation, which remove suspended solids
(turbidity) from water by causing the suspended particles to aggregate into a
slime, that settles out of the water.
This technique is used in
treating wastewater, both industrial and treated sewage, from municipal
wastewater treatment plants.
It is also used, as a first
step, in treating raw water for industrial use, and in food and beverage
production.
THE PROCESS
Coagulation uses salts such as aluminum sulfate (alum) or ferrous of ferric (iron) salts, which bond to the suspended particles, making them less stable in suspension, i.e., more likely to settle out.
Coagulation uses salts such as aluminum sulfate (alum) or ferrous of ferric (iron) salts, which bond to the suspended particles, making them less stable in suspension, i.e., more likely to settle out.
Flocculation is the binding
or physical enmeshment of these destabilized particles, and results in a slime
that is heavier than water, which settles out in a clarifier.
Flocculation agents are
natural synthetic polymers and synthetic organic polymers used to form the flocculant.
BACKGROUND
Water is clarified using the processes of
coagulation and flocculation, which remove suspended solids (turbidity) from
water by causing the suspended particles to aggregate into a slime, that
settles out of the water.
This technique is used in treating wastewater,
both industrial and treated sewage, from municipal wastewater treatment plants.
It is also used, as a first step, in treating
raw water for industrial use, and in food and beverage production.
THE PROCESS
Coagulation uses salts such as aluminum sulfate
(alum) or ferrous of ferric (iron) salts, which bond to the suspended
particles, making them less stable in suspension, i.e., more likely to settle
out.
Flocculation is the binding or physical
enmeshment of these destabilized particles, and results in a slime that is
heavier than water, which settles out in a clarifier.
Flocculation agents are natural synthetic
polymers and synthetic organic polymers used to form the flocculant.
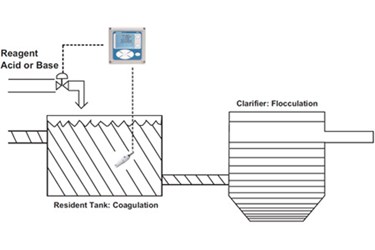
pH EFFECTS
The salts used for coagulation form certain ions
in solution that are responsible for the coagulation action taking place.
However, the actual ions produced by these salts
depend upon the pH of the water sample. At varying sample pH values, the
coagulation process may suffer from less than optimum ions being formed in
solution.
pH that is too low may not allow the coagulation
process to proceed, while high pH can cause a coagulated particle to
redisperse.
The size of the coagulated particles is also
affected by pH, which, in turn, determines the density of the flocculated slime
and its tendency and rate of settling out.
The optimum pH for the coagulation and
flocculation process must be determined experimentally.
It is specific to each application and is
dependent upon the sample, the coagulation and flocculation agents used, the
desired clarity of the water, and the water’s end use.
pH MONITORING AND CONTROL
pH control of the sample prior to clarification
will also vary from application to application.
An industrial waste sample, for instance, may be
subject to wider pH variations, requiring stepwise pH control, while a raw
water sample for beverage production may only need small adjustments for minor
variations in pH and alkalinity.
Clarification is typically either the first step
in treating raw water, often river water or even city (potable) water, or the
last step that is prior to discharge of a wastewater sample.
As a result, the demands on the pH sensor, in
terms of temperature, pressure, and corrosion resistance, are minimal.
In certain cases, a pH measurement may be
required in the clarifier itself or after the clarifier for subsequent pH
adjustment.
The pH sensor may be exposed to the flocculant
(flocculating polymer and slime), which can form a tenacious coating on the pH
sensor.
Since the polymer coating is organic in nature,
it can be readily cleaned off using a solvent suitable for the polymer, but
which does not attack the sensor’s materials of construction.
The 396P TUpH™ Sensor, which resists the effects
of coating, can be used to maximize the time between required cleanings.
™ TUpH is a registered trademark of Rosemount Analytical, Uniloc Division.

No comments:
Post a Comment