The Virginia Cooperative
Extension (VCE) Offices in Virginia occasionally holds drinking water clinics
for well, spring and cistern owners as part of the Virginia Household Water
Quality Program. The VCE subsidizes the analysis cost for these clinics. Currently,
samples are analyzed for: iron, manganese, nitrate, lead, arsenic, fluoride,
sulfate, pH, total dissolved solids, hardness, sodium, copper, total coliform
bacteria and E. Coli bacteria at a cost of $49 to the well owner.
This is far from an
exhaustive list of potential contaminants, but with one or two exceptions these
are the most common contaminants that effect drinking water wells. These are
mostly the naturally occurring contaminants and common sources of
contamination: a poorly sealed well or a nearby leaking septic system, or
indications of plumbing system corrosion.
There are other
contaminants that can be found in ground water in certain regions that can
cause illness when exposed to small amounts over long periods of time. Uranium
is an example.
There are also
nuisance contaminants for which there is not an approved EPA methodology. Iron
bacteria is an example.
A through water
analysis should be performed before any treatment is considered to make sure
the selected treatment is necessary and appropriate.
Wells should be
tested annually for bacteria and every 1-3 years for other common contaminants
especially if you install treatment systems.
Groundwater is
dynamic and can change over time. And it is important to make sure that any
treatment is still appropriate and effective.
Water treatment systems are not an
install-and-forget piece of equipment. They are more of systems to maintain,
adjust and control to keep the water within ideal parameters.
Improperly treated
water can be as problematic as not treating water.
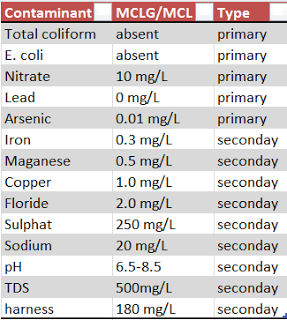
In order to determine if treatment is necessary, water test results
should be compared to a standard. The standard we use if the U.S. EPA Safe
Drinking Water Act in the list to the left.
There are primary and secondary drinking water
standards.
Primary standards are ones that can impact
health and from the list above include: coliform bacteria, E. coli and fecal
coliform bacteria, nitrate, lead, and arsenic.
Groundwater can
sometimes be contaminated from nearby or historic land use.
Before a home is
purchased, a much more comprehensive water analysis should be performed to
ensure that groundwater is not contaminated with hydrocarbons, solvents, fuels,
heavy metals, pesticides.
Coliform bacteria are
not a health threat itself - it is used to indicate other bacteria that may be
present and identify that a well is not properly sealed from surface bacteria.
The federal standard
for coliform bacteria is zero, but the federal standard allows that up to 5% of
samples can test positive for coliform during a month. New coliform standards
are anticipated to be promulgated shortly.
Fecal coliform and
E. coli are bacteria whose presence indicates that the water is contaminated
with human or animal wastes.
Disease-causing
microbes (pathogens) in these wastes can cause diarrhea, cramps, nausea,
headaches, or other symptoms.
.
These pathogens may pose a special health risk for infants, young children, and those with compromised immune systems.
.
These pathogens may pose a special health risk for infants, young children, and those with compromised immune systems.
However, people can
drink water contaminated with fecal bacteria and not notice.
If your water is
contaminated with coliform but not fecal coliform or E. coli, then you have a
nuisance bacteria problem and the source may be infiltration from the surface
from rain or snow melt.
Typical causes are
improperly sealed well cap, failed grouting or surface drainage to the well.
Shock chlorinate the
well, repack the soil around the well pipe to flow away from the well and
replace the well cap.
Then after the next
big rainstorm retest the well for coliform. If it is still present then a
long-term treatment should be implemented: using UV light, ozonation, or
chlorine for continuous disinfection.
If you have fecal
coliform in the well or E. coli, your well is being impacted by human or animal
waste.
If there is not a
nearby animal waste composting facility, then you are probably drinking water
from a failed septic system - yours or your nearest neighbors’.
To solve this
problem you need to either fix or replace the septic system that is causing the
contamination or replace the well. The failing septic systems can often be
identified by using tracer dyes.
While continuous
disinfection will work to protect you from fecal bacteria and E. coli, be aware
that if your well is being impacted by a septic system, then the well water
might also have present traces of all the chemicals and substances that get
poured down the drain.
Long term treatment
for disinfection, and micro-filtration should be implemented: using UV
light, ozonation, or chlorine for continuous disinfection and carbon filtration.
Anything that is
used for drinking should be further treated with a reverse osmosis systems or
micro membrane system that work by using pressure to force water through a
semi-permeable membrane.
This is the type of
system that is used to desalinate water. Large quantities of wastewater are
produced by reverse osmosis systems and need to bypass the septic system or
they will overwhelm that system creating more groundwater problems.
Reverse osmosis
systems produce water very slowly, a pressurized storage tank and special
faucet needs to be installed so that water is available to meet the demand for
drinking and cooking.
Nitrate can
contaminate well water from fertilizer use; leaking from septic tanks, sewage
and erosion of natural deposits.
The MCL for nitrate
is 10 mg/L. Infants below the age of six months who drink water containing
nitrate in excess of the MCL could become seriously ill from blue-baby syndrome
and, if untreated, may die.
Symptoms include
shortness of breath and a blue ting to the skin common in blue-baby syndrome.
The NO3 dissolves and moves easily through soil which varies seasonally and
over time as plants use up the nitrate over the summer.
Testing in the
spring will usually produce the highest levels. Nitrate may indicate
contamination from septic tanks, but do not boil the water- boiling water
reduces the water and actually INCREASES the concentration of nitrates.
So if your water is
being impacted by a septic system and you do not replace the well;
distillation, reverse osmosis, or ion exchange is necessary to control the
nitrate.
The
EPA guidance for sulfate is 250 ppm for taste.
Sulfates can clog plumbing and
stain clothing and excessive levels can have a laxative effect.
Hydrogen sulfide naturally
occurs in shale, sandstone, and near coal or oil fields.
Sulfate and hydrogen
sulfide are not regulated by the EPA for drinking water, they are a secondary
contaminant and though extremely unpleasant, harmless to animals, but not to
plumbing equipment.
There is a related
problem (for which there are limited methods of testing) of sulfur reducing
bacteria. According to the EPA, sulfur-reducing bacteria and sulfur-oxidizing
bacteria pose no known health risks.
Sulfur-reducing
bacteria live in oxygen-deficient environments such as deep wells, plumbing
systems, water softeners, and water heaters. These bacteria usually flourish in
hot water tanks and pipes.
Sulfate reduction
can occur over a wide range of pH, pressure, temperature, and salinity
conditions and produce the rotten egg smell and the blackening of water and
sediment by iron sulfide. Sulfate-reducing bacteria can cause the corrosion of
iron in pipes and water systems.
The treatment method
selected depends on many factors including the level of sulfate in the water,
the amount of iron and manganese in the water, and if bacterial contamination
also must be treated.
High concentrations
of dissolved hydrogen sulfide also can foul the resin bed of an ion exchange
water softener.
When a hydrogen
sulfide odor occurs in treated water (softened or filtered) and no hydrogen
sulfide is detected in the non-treated water, it usually indicates the presence
of some form of sulfate-reducing bacteria in the system.
Water softeners
provide an environment for these bacteria to grow. “salt-loving” bacteria, that
use sulfates as an energy source, may produce a black slime inside water
softeners.
If you have modest
sulfate, but no rotten egg smell, installing a water softening system may
create additional problems, especially if the system is not meticulously
maintained.
If you have a rotten
egg smell associated with the hot water and elevated levels of sulfate on the
cold water side, your hot water tank may be fouled with sulfur reducing
bacteria, or the tank’s corrosion control rod may be causing the sulfur to
react in the heated environment.

At naturally
occurring levels, iron and manganese do not present a health hazard.
However,
their presence in well water can cause unpleasant taste, staining and
accumulation of mineral solids that can clog water treatment equipment and
plumbing.
The standard
Secondary Maximum Contaminant Level (SMCL) for iron is 0.3 milligrams per liter
(mg/L or ppm) and 0.05 mg/L for manganese.
This level of iron and manganese are
easily detected by taste, smell or appearance.
In
addition, some types of bacteria react with soluble forms of iron and manganese
and form persistent bacterial contamination in a well, water system and any
treatment systems.
These organisms
change the iron and manganese from a soluble form into a less soluble form,
thus causing precipitation and accumulation of black or reddish black or reddish brown gelatinous material (slime).
All systems of
removing iron and manganese essentially involve oxidation of the soluble form
or killing and removal of the iron bacteria.
When the total
combined iron and manganese concentration is less than 15 mg/l, an oxidizing
filter is the recommended solution.
An oxidizing filter supplies oxygen to convert
ferrous iron into a solid form which can be filtered out of the water.
Higher concentrations of iron and manganese can be
treated with an aeration and filtration system.
This system is not
effective on water with iron/ manganese bacteria, but is very effective on
soluble iron and manganese.
Chemical oxidation can be used to remove high
levels of dissolved or oxidized iron and manganese as well as treat the
presence of iron/manganese (or even sulfur) bacteria.
The system consists
of a small pump that puts an oxidizing agent into the water before the pressure
tank.
The water will need about 20 minutes for oxidation to take place so
treating before a holding tank or pressure tank is a must.
After the solid
particles have formed the water is filtered.
The best oxidizing
agents are chlorine or hydrogen peroxide. If chlorine is used, an activated
carbon filter is often used to finish the water and remove the chlorine taste.
The holding tank or
pressure tank will have to be cleaned regularly to remove any settled particles.
Fluoride occurs
naturally in groundwater and in certain parts of Eastern Virginia there are
very high naturally occurring levels.
Fluoride is a primary water contaminant
and the EPA MCL 4.0 mg/L and SMCL 2.0 mg/L.
Fluoride is
typically added in small quantities to public water supplies the optimum
concentrations for public systems 0.8 - 1.2 mg/L. Excessive levels of fluoride
can cause fluorosis or bone cancer over long term exposure.
Treatment for
excessive levels of fluoride in water is typically reverse osmosis which will
remove all fluoride and minerals from water.
The pH of water is a
measure of the acidity or alkalinity.
The pH is a logarithmic scale from 0 – 14
with 1 being very acidic and 14 very alkaline.
Drinking water should be between
6.5 and 7.5.
For reference and to put this into perspective, coffee has a pH of
around 5 and salt water has a pH of around 9.
Corrosive water, sometimes also
called aggressive water is typically water with a low pH. (Alkaline water can
also be corrosive.)
Low pH water can
corrode metal plumbing fixtures causing lead and copper to leach into the water
and causing pitting and leaks in the plumbing system.
The presence of lead
or copper in water is most commonly leaching from the plumbing system rather
than the groundwater.
Acidic water is easily treated using an acid neutralizing
filter.
Typically these
neutralizing filters use a granular marble, calcium carbonate or lime.
If the
water is very acidic a mixing tank using soda ash, sodium carbonate or sodium
hydroxide can be used.
The acid
neutralizing filters will increase the hardness of the water because of the
addition of calcium carbonate. The sodium based systems will increase the salt
content in the water.
Water that contains
high levels of dissolved minerals is commonly referred to as hard.
Groundwater
very slowly wears away at the rocks and minerals picking up small amounts of
calcium and magnesium ions.
Water containing
approximately 125 mg/L can begin to have a noticeable impact and is considered
hard. Concentration above 180 mg/L are considered very hard.
As the mineral
level climbs, bath soap combines with the minerals and forms a pasty scum that
accumulates on bathtubs and sinks.
You either must use
more soap and detergent in washing or use specially formulated hard water soap
solutions.
Hard water can be just a minor annoyance with spotting and the
buildup of lime scale, but once water reaches the very hard level 180 mg/L or
10.5 grains per gallon, it can become problematic.
Hard water spots
appear on everything that is washed in and around the home from dishes and
silverware to the floor tiles and cars.
When heated calcium carbonate and
magnesium carbonate are removed from the water and form a scale (lime scale) in
cookware, hot water pipes, and water heaters.
Water softening systems are used to address the
problem are basically an ion exchange system.
The water softening system consists of a mineral tank and a brine tank.
The water supply
pipe is connected to the mineral tank so that water coming into the house must
pass through the tank before it can be used.
The mineral tank
holds small beads of resin that have a negative electrical charge.
The calcium and magnesium ions are
positively charged and are attracted to the negatively charged beads.
This attraction makes the minerals stick to the
beads as the hard water passes through the mineral tank.
Sodium is often used
to charge the resin beads.
As the water is softened, the sodium ions are
replaced and small quantities of sodium are released into the softened water,
thus the salty taste of softened water.
When the water softening system is recharged the
excess sodium solution carrying the calcium and magnesium is flushed to the
septic system which may shorten the life of the drain field.
At the present time
the EPA guidance level for sodium in drinking water is 20 mg/L.
This level was developed for those restricted to a total sodium intake of
500 mg/day and does not necessarily represent a necessary level for the rest of
the population.
Based on taste of
the water levels of sodium should be below 30 to 60 mg/L based on individual
taste. Water softening systems add sodium.
Reverse osmosis
systems and distillation systems remove sodium and are safe for household use,
but addressing hard water by using vinegar to descale pots and dishwashers,
regularly draining hot water heaters, and using detergents formulated for hard
water might be a better solution for you.
Arsenic
is not a common contaminant in groundwater that has not been impacted from
surface runoff.
Arsenic can be
caused by erosion of natural deposits, but is more typically caused by
runoff from orchards, runoff from glass & electronics production wastes, or
leaching from coal ash disposal of or agricultural chemical mixing areas.
The EPA standard for
arsenic is 0.01 mg/L. Arsenic removal depends on the type of arsenic (there are
two types) and the other contaminants present in water.
Arsenic removal
methods or systems include anion exchange, reverse osmosis, activated alumina,
and other types of adsorptive media filters.
Each method has its limitations,
advantages and disadvantages and should be chosen based on additional analysis.
Elizabeth Ward was awarded an MBA from the University of Pittsburgh and an MS ChE
from Polytechnic Institute of NYU, worked as a chemical engineer for both the
US EPA in DC, and at DuPont before working in finance and then becoming
consultant with Washington Advisors and is the author of "The Lenders
Guide to Developing an Environmental Risk Management Program." Elizabeth
retired from Washington Advisors and began her volunteer career and is
currently the Treasurer of the Prince William Soil and Water Conservation
District.
You might also like:
.
 |
Multi-Media Filter, Highly-Activated Carbon Filter,
Zeolite-Process Water Softener With Brine Tank,
Fiberglass Ballast-Type Pressure Tank
(fully automatic backwash & regeneration)
|
PURICARE
Water
Treatment
Systems
.
.
...
Aganan, Pavia, Iloilo, Philippines
...
CLICK HERE . . . to view company profile . . .











No comments:
Post a Comment