 |
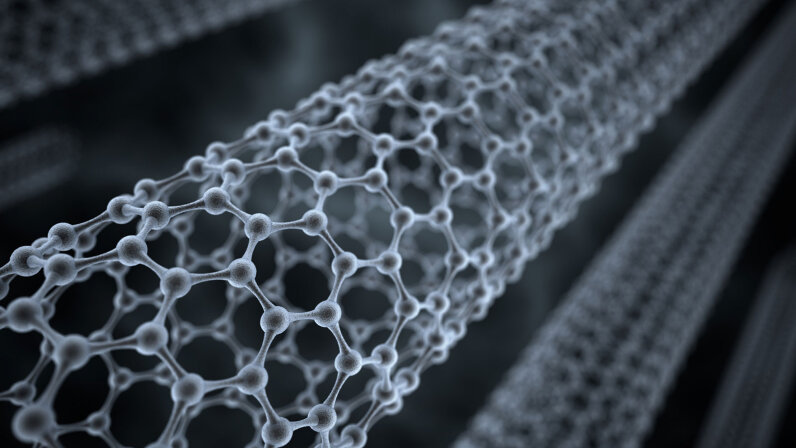
A sheet of graphene looks like an atomic-scale honeycomb.
|
200
Times Stronger Than Steel, 1,000 Times Lighter Than Paper
BY
DAVE ROOS
In 2004, two scientists at the University of
Manchester conducted a deceptively simple experiment with potentially
world-changing consequences.
The researchers, Andre Geim and Konstantin
Novoselov, were playing around with graphite, the stuff in the tip of your
pencil.
Graphite is made of super-thin sheets of pure
carbon stacked on top of each other.
Geim and Novoselov wanted to see if they
could isolate a single sheet of graphite, an impossibly thin layer of carbon
measuring just one atom thick.
So, they grabbed a roll of sticky tape. Yes,
the same transparent tape in the plastic applicator that you keep in your junk
drawer.
Here's how Geim described his technique, as
reported by the BBC.
"You put [sticky
tape] on graphite or mica and peel the top layer. There are flakes of graphite
that come off on your tape. Then you fold the tape in half and stick it to the
flakes on top and split them again. And you repeat this procedure 10 or 20
times. Each time, the flakes split into thinner and thinner flakes. At the end
you're left with very thin flakes attached to your tape. You dissolve the tape
and everything goes into solution."
The sticky-tape method worked!
By isolating a single-layer sheet of carbon,
Geim and Novoselov were credited with discovering a brand-new material called
graphene that's now believed to be the strongest, lightest and most
electrically conductive substance on Earth.
In 2010, Geim and Novoselov shared the Nobel
Prize in physics for discovering graphene, and researchers around the world
began clamoring for ways to use this remarkable "supermaterial" to
build more powerful and longer-lasting batteries, faster microchips, flexible
circuitry, implantable biosensors and more.
A decade later, graphene has yet to deliver
on its much-hyped promises, but insiders are confident that we'll finally be
seeing smartphones, electric cars and sensors using graphene-based technology
in the next few years.
Why Is Graphene a Supermaterial?
Measuring in at just one atom thick, a sheet
of graphene checks all the boxes of a supermaterial:
o Graphene is 200 times
stronger than steel by weight.
o It is 1,000 times
lighter than paper.
o It is 98 percent
transparent.
o It conducts
electricity better than any other known material at room temperature.
o It can convert light
at any wavelength into a current.
o And, last but not
least, graphene is made from carbon, the fourth most-abundant element in the
universe, so we're not likely to run out
Graphene gets its superpowers from its
structure.
If you could zoom in close enough, you'd see
that a sheet of graphene looks like an atomic-scale honeycomb.
Individual carbon atoms are arranged in a
hexagonal pattern that resembles chicken wire.
Each carbon atom in a sheet of graphene is
covalently bonded to three other carbon items, which gives the material its
incredible strength.
Why does graphene conduct electricity so
well?
Again, because of the way those carbon atoms
are bonded.
Each carbon atoms has four electrons in its
outer shell, but only three of those electrons are shared with its neighboring
three carbon atoms.
The remaining electron is called a pi
electron and is free to move in three-dimensional space, which allows it to
transmit electrical charges across the sheet of graphene with almost no
resistance. In fact, graphene is the fastest conductor of
electricity at room temperature of any known substance.
The 'Magic Angle'
A recent discovery may add yet another
superpower to graphene's brag list.
A team at Massachusetts Institute of
Technology (MIT) was experimenting with dual-layered graphene — two layers of
single-atom graphene stacked together — when they stumbled upon a new, nearly
magical property of graphene.
When the layers are rotated slightly out of
line with each other — a displacement of exactly 1.1 degrees — the graphene
becomes a superconductor.
Superconductors are the rarest class of
materials that conduct electricity with absolutely no resistance and zero heat.
The discovery of graphene's "supermaterial" sent shockwaves through the scientific community.
Although the experiment was conducted at
extreme low temperatures (close to 0 degrees Kelvin or minus 459.67 F), it
opened up the possibility that by combining graphene with other superconductive
elements, we're closer than ever to room-temperature superconductivity.
Such an achievement would radically improve
the energy efficiency of everything from gadgets to cars to entire electric
grids.
How Might Graphene Transform Technology?
Superconductivity is still decades away, but
revolutionary graphene-based products are coming to the market much sooner,
says Andrea Ferrari, a professor of nanotechnology and director of the
Cambridge Graphene Centre.
"By 2024, there
will be a variety of graphene products on the market," says Ferrari, "including
batteries, photonics, night vision cameras and more."
Consumers have been eagerly awaiting
graphene-based batteries for years.
The lithium-ion batteries in all our gadgets
are relatively slow to charge, lose their juice quickly and burn out after a
set number of cycles.
That's because the electrochemical process
that powers lithium-ion batteries generates a lot of heat.
But since graphene is the world's most
efficient electrical conductor, it produces a lot less heat when charging up or
discharging electricity.
Graphene-based batteries are promising five
times faster charging speeds than lithium-ion, three times longer battery life,
and five times as many cycles before they need to be replaced.
Electronics companies like Samsung and Huwei
are actively developing graphene-based batteries for smartphones and other
gadgets, but the earliest those will hit the market is 2021.
As for graphene batteries in electric cars —
which could dramatically increase their driving radius — that's still a few
years off.
An entire industry has been built on
lithium-ion technology and it won't change overnight.
"The battery
industry is very conservative," says Jesus de la Fuente, CEO of Graphanea, a
company that manufactures and sells pure graphene and graphene-based chips to
academic researchers and R&D departments.
"It might change
the composition of batteries a few times every five to ten years, which makes
it very difficult to introduce new products in this industry."
There are a few graphene-based batteries on
the market, including some wired and wireless chargers from a company called
Real Graphene, but those are only the tip of the iceberg, says Ferrari, who is
also the science and technology officer for the Graphene Flagship, a
1-billion-euro collaboration by the European Union to speed the development of
graphene technology.
Research partners with the Flagship are
already making graphene batteries that outperform today's best high-energy
cells by 20 percent capacity and 15 percent energy.
Other teams have built graphene-based solar
cells that are 20 percent more efficient at converting sunlight to electricity.
Other Uses for Graphene
While graphene batteries might be first to
market, researchers are busy developing countless other applications for this
miracle material.
Biosensors are a big deal. Imagine an
incredibly thin and flexible chip that can be injected into the bloodstream to
monitor real-time health data like insulin levels or blood pressure.
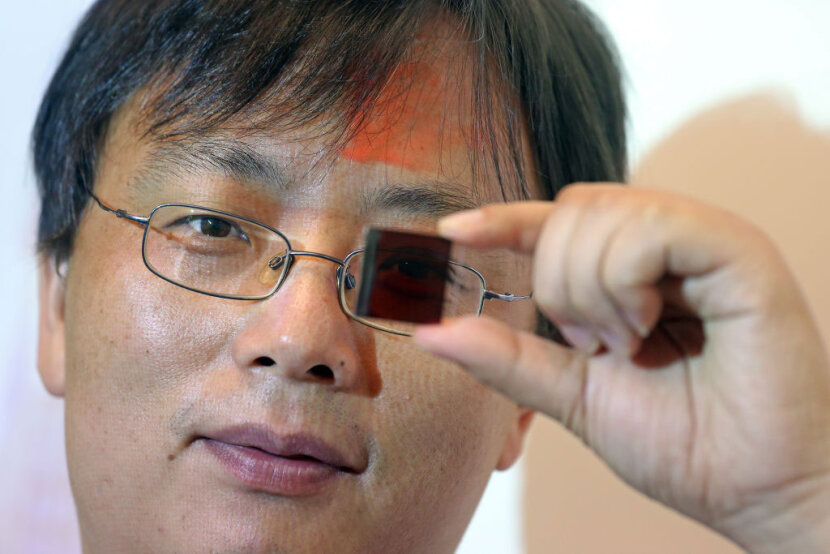 |
| Physics professor Dr. Yan Fung, holds a new invention of low-cost semitransparent solar cells with graphene electrodes at Poly University in Hung Hom, China, 2015. |
Thin, stretchable sensors can also be worn on
the skin or woven into the fabric of clothes.
Photonics is another field that's already
incorporating graphene.
By integrating graphene into light-sensitive
chips, cameras and other sensors can greatly improve sensitivity to even the
faintest light waves across the visible and invisible spectrum.
That will not only improve the image quality
of cameras and telescopes, but also medical imagery.
Filtration is yet another promising
application of graphene.
Simple water purification filters built with
graphene polymers can bind to organic and inorganic contaminants in drinking
water.
Researchers at the Graphene Flagship have
also created desalinization technologies based on graphene diodes that can
remove over 60 percent of salt from seawater for agricultural and other uses.
All these developments will take time, but
Ferrari at the Cambridge Graphene Centre is confident that graphene will live
up to its hype.
In fact, he is equally excited about the
yet-undiscovered properties of the estimated 2,000 other monolayer materials
that are also being isolated, sticky tape method or otherwise.
"We say
graphene, but we're really talking about a large number of options that are
being explored,"
says Ferrari. "Things are moving in the right direction."
NOW THAT'S COOL
Sports equipment maker Head was one of the
first to jump on the graphene bandwagon. Its Graphene XT tennis racket claims
to be 20 percent lighter than racquets with the same swing weight.
Dave
Roos
CONTRIBUTING
WRITER
Dave
is a freelance journalist who has contributed hundreds of articles to
HowStuffWorks since 2007, with a specialty in personal finance, economics and
business. Raised in Pittsburgh, Pennsylvania, he attended Duke University where
he earned the B.A. in comparative religious studies that has served him so
well.
Dave
began freelancing when he and his wife moved to Mexico in 2003, publishing
articles about Mexican food and culture in The New York Times, the Los Angeles
Times and Newsweek. Nearly 15 years and three kids later, Dave and his family
recently moved back to Mexico and just might stay a while.


No comments:
Post a Comment